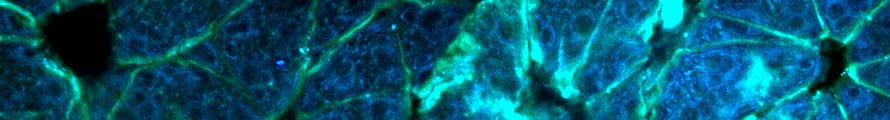
The Imaging and Spectroscopy core is primarily located at the Engineering Research Center (ENRC) with a satellite facility available on campus adjacent to the Bioenergetics Core. These facilities currently occupy an area of over 3,000 sq. ft.
State-of-the-Art Microscopy Equipment Available:
Other Free-to-use Equipment:
- Hamamatsu NanoZoomer
- High-speed whole slide imaging system
- Quantitative polarized light imaging (QPLI) microscope
- Capable of quantifying collagen fiber orientation and thickness
- Cryostat
- Handheld diffuse reflectance spectroscopy (DRS) system
- Karl Storz veterinary endoscopy system
- Raman spectrometer
Services:
- Equipment training
- Study design conceptualization
- Data acquisition
- Data analysis for multiphoton microscope images
- Optical redox ratio
- Collagen fiber second harmonic generation (SHG)
- Fiber orientation
- Fiber organization
- Fiber tracking over time
- NADH fluorescence lifetime
- Bi-exponential fitting
- Phasor analysis
- Mitochondrial clustering
- Data visualization and statistical analysis
Please contact Core personnel to schedule access to equipment or discuss experiment plans.
Core Co-Directors

Dr. Narasimhan Rajaram
Associate Professor
Biomedical Engineering
College of Engineering
University of Arkansas
nrajaram@uark.edu
Profile

Dr. Timothy Muldoon
Associate Professor
Biomedical Engineering
College of Engineering
University of Arkansas
tmuldoon@uark.edu
Profile
Core Manager

Dr. Alan Woessner
Research Associate
Biomedical Engineering
College of Engineering
University of Arkansas
aewoessn@uark.edu
Profile