Needle-Based Fluorescence Lifetime Imaging (FLIM) for Muscle Loss and Regeneration
This needle-based FLIM system allows for minimally invasive monitoring of host cell activation and proliferation in a rodent model of volumetric muscle loss (VML) repair.
Figure 1. Needle-based FLIM system placed into the left tibialis anterior (TA) muscle.
Spectroscopy for Monitoring Progression of Ulcerative Colitis
Ulcerative colitis (UC) is a gastrointestinal, autoimmune disease that causes ulceration and inflammation of the colon with high incidence in North America and Western Europe. In order to determine the cellular mechanism behind relapse and remission of UC, researchers employ destructive analysis methods such as immunohistochemistry, western blotting, and gene sequencing. There is an emerging interest in using non-invasive techniques to study the in vivo, longitudinal effects of UC on the mucosa of the colon. We have developed a mouse model of UC and have employed existing spectroscopy methods to study the changes in tissue hemodynamics during the progression of UC.
Figure 2. A) Normal mucosa B) DSS-induced ulcer in the mucosa C)DRS acquisition of an ulcer in a murine model of UC (yellow arrow indicates probe placement on ulcer)
Tomographic Microendoscopy for Characterization of Epithelial Tissue Structure and Function
This proposal aims to develop a multi-projection transport-based reflectance image mapping microendoscopy system with a small diameter and no moving parts or complex scanning mechanisms. Tomographic three-dimensional reconstruction of absorption or fluorescence data is possible using a fiber bundle microendoscopy architecture by spatial
mapping of this remitted light (surface flounce), captured via the fiber bundle. This novel approach can have a transformational impact in a number of basic science and clinical applications, including the study of more physiologically relevant orthotopic models of disease -such as colorectal cancer- and clinical assessment of patient-specific tumor phenotype and tumor vasculature for personalized therapy in anatomical locations currently inaccessible by current endoscopic technologies.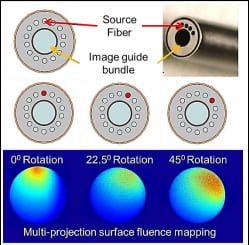
Figure 3.Individually addressable peripheral illumination fibers enable multi-projection surface measurements.
Multiphoton Imaging for Monitoring Macrophage Activation and Function
Using two-photon microscopy and endogenous fluorescence, we are investigating changes in murine (mouse) macrophages during cytokine stimulated activation and in co-cultured with murine cancer cells. This approach utilizes a myriad of optical markers acquired through label-free two-photon microscopy such as collagen structure, metabolic ratios, and spectral data.