Agreeing that humans have drastically altered our earth’s climate by releasing massive amounts of Greenhouse Gases (GHG) into the atmosphere is a no-brainer. However, understanding the individual effect each of these gases has on Climate Change can be a bit more difficult.
Not all GHGs are created equal.
In fact, all of the GHGs trap heat at different rates and have different lifetimes in the atmosphere, making their contributions to the Greenhouse Effect extremely diverse. How then, can we effectively compare the impact of these six gases in our quest to combat Climate Change? The answer lies in the Global Warming Potential (GWP).
Global Warming Potential is a measurement of how much heat a GHG traps in the atmosphere over a specific amount of time as compared to CO2.
 Carbon Dioxide, CO2, is used as a benchmark for this measurement, so its heat trapping capability is rated at 1. All other gases are represented in comparison to this value.
Carbon Dioxide, CO2, is used as a benchmark for this measurement, so its heat trapping capability is rated at 1. All other gases are represented in comparison to this value.
For example, if one kilogram (yes, kilogram – GWPs are represented in metric) of methane, CH4, over 100 years is given a GWP of 25, this means that one kilo of CH4 will trap 25 times more heat in our atmosphere than one kilo of CO2 would over the same time.
This chart shows the six Kyoto Protocol GHGs with their GWPs.
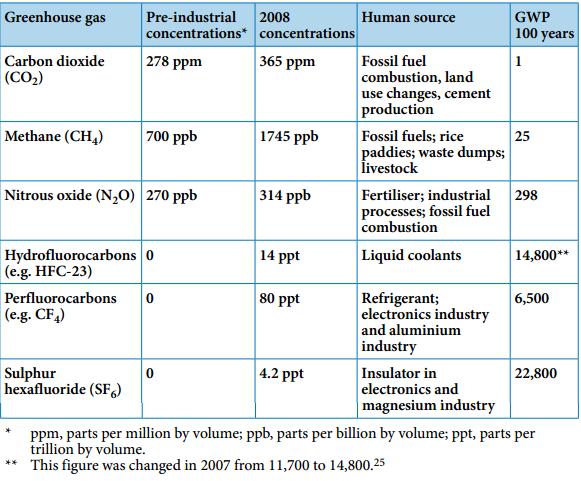
chart from http://www.fern.org/book/trading-carbon/box-2-difficulty-measuring-greenhouse-gases
GWP is also useful in calculating and comparing the CO2e of GHGs.
CO2e (also sometimes written as “CO2eq”, “CO2equivalent”, or even “CDE”) shorthand for Carbon Dioxide Equivalent, is basically the unit used to represent the “warming effect” of a specific batch of gas. Like GWP, CO2e uses carbon as a benchmark of 1, creating a unit that any GHG can be converted into. To find the CO2e of a specific amount of gas, say 10 tonnes (metric again) of Nitrous Oxide, you simply multiply the quantity of gas by its GWP.
Ammount of GHG x GWP = CO2e
As mentioned above, CO2e’s are especially handy for comparing the warming impact of two different batches of gas that would otherwise be hard to relate.
For example, I can easily compare the warming effect of 10 tonnes of Sulfur hexafluoride and 1000 tonnes of Nitrous Oxide by calculating their CO2e’s.
 10 tonnes SF6 x 22,800 = 228,000 CO2e
10 tonnes SF6 x 22,800 = 228,000 CO2e
1000 tonnes N2O x 298 = 298,000 CO2e
Finding the CO2e of these batches of gases shows me that even though I have 100 times more N2O than SF6, Sulfur hexafluoride is such a powerful GHG that the warming effect of the two batches is roughly the same.
When understood correctly, both GWP and CO2e are handy tools for comparing and communicating the warming effects of different GHGs.
Part 2 in a series on sustainability definitions. Click here to read other posts in this series.